Even when a person suffering from malaria is burning up with fever and too sick to function, the tiny blood-eating parasites lurking inside them continue to flourish, relentlessly growing and multiplying as they gobble up the host’s red blood cells.
The single-celled Plasmodium parasites that cause 200 million cases of malaria each year can withstand feverish temperatures that make their human hosts miserable. And now, a Duke University-led team is beginning to understand how they do it.
Assistant professor of chemistry Emily Derbyshire and colleagues have identified a lipid-protein combo that springs into action to gird the parasite’s innards against heat shock.
Understanding how the malaria parasite protects its cells against heat stress and other onslaughts could lead to new ways to fight resistant strains, which have evolved ways to survive the drugs traditionally used to kill them, the researchers say.
Nearly half of the world’s population is at risk of contracting malaria. The disease kills 400,000 people a year, most of them children.
Long before the cause of malaria was identified, the disease’s harrowing fevers were well known. References to them have been found on 5,000-year-old clay tablets from ancient Mesopotamia. The Greek poet Homer wrote about their misery. Hippocrates too.
The Duke team, collaborating with professor of biological engineering Jacquin Niles at the Massachusetts Institute of Technology, wanted to know how the malaria parasites inside a person’s body make it through these fevers unscathed.
When the parasites enter a person’s bloodstream through the bite of an infected mosquito, the temperature around them jumps from the balmy mid-70s of the mosquito to 98.6 degrees in the human. The human host’s body temperature can then rocket to 105 degrees or higher before dropping back down to normal two to six hours later, a roller coaster pattern that repeats itself every two to three days.
“It’s like going from room temperature water to a hot tub,” said first author Kuan-Yi Lu, who earned his Ph.D. in molecular genetics and microbiology in Derbyshire’s lab at Duke.
For the paper, published Sept. 25 in the journal eLife, Lu spent hundreds of hours peering at parasites under the microscope, trying to figure out what happens inside them when temperatures seesaw.
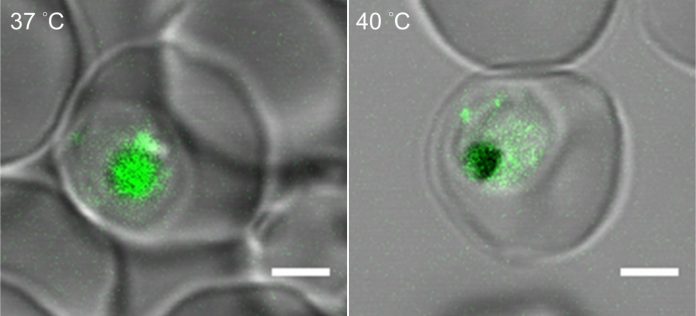
To mimic malarial fever in the lab, the researchers placed malaria-infected red blood cells in an incubator heated to 104 degrees Fahrenheit for six hours before bringing them back down to normal body temperature, 98.6 degrees.
They found that when temperatures rise, the parasites produce more of a lipid molecule called phosphatidylinositol 3-phosphate, or PI(3)P.
This substance builds up in the outer wall of a tiny sac inside the parasite’s cells called the food vacuole — the protist’s version of a gut. There, it recruits and binds to another molecule, a heat shock protein called Hsp70, and together they help shore up the food vacuole’s outer walls.
Without this lipid-protein boost, the team found that heat can make the food vacuole start to leak, unleashing its acidic contents into the gel-like fluid that fills the cell and possibly even digesting the parasite from the inside.
The findings are important because they could help researchers make the most of existing malaria drugs.
Previous research has shown that malaria parasites with higher-than-normal PI(3)P levels are more resistant to artemisinins, the leading class of antimalarials. Since artemisinins were first introduced in the 1970s, partial resistance has been increasingly reported in parts of Southeast Asia, raising fears that we may be losing one of our best weapons against the disease.
But the Duke-led study raises the possibility that new combination therapies for malaria — artemisinins combined with other drugs that reduce the parasite’s PI(3)P lipid levels and disrupt the food vacuole’s membrane — could be a way to re-sensitize resistant parasites, breaking down their defenses so the malaria treatments we already have are effective again.
“If there is an alternative way to increase the permeability of the digestive vacuole, it could make the digestive vacuole more accessible to those drugs again,” Lu said.
The findings also suggest caution in giving malaria patients ibuprofen for fever if they’re already taking artemisinin-based compounds, Derbyshire said. That’s because artemisinins kill malaria parasites by damaging their cell’s survival machinery, including the machinery that makes PI(3)P. If artemisinins suppress PI(3)P levels, and thereby make malaria parasites more vulnerable to heat stress, then fever reducers could prolong the time it takes for artemisinin-based drugs to kill the parasites, as some reports have suggested.
Much remains to be learned, Derbyshire said. “There’s more work to do to establish the mode of action. But you could imagine designing new combination therapies to try and extend the life of artemisinin and prolong its effectiveness,” Derbyshire said.